
EV Battery Overview
In our previous article, we discussed the advantages of owning an electric vehicle. Now, let’s delve further into the component of an EV – its battery.
Electric vehicle batteries consist of several subcomponents that work together to store and discharge electricity. These individual sections are also known as cell components or cell materials. The parts combine to form the complete battery and each has its unique properties and function.
When considering the various types of electric car batteries, it’s important. Knowing how they function can help you make a more informed decision when purchasing a new electric car, hybrid, or extended-range electric vehicle (EREV) battery.
What are the Components of EV Batteries?
Before we review these components, we need to make sure we understand what an electrode is.
An electrode is a conductor which is a negatively charged (anode) or a positively charged (cathode) material. You can read more about electrodes here.
The different elements of an electric car battery include the following:
-
- Anode – The anode is the negative electrode of the battery. It’s made from a metallic oxide material, such as nickel oxide or iron oxide. Anodes are highly porous, allowing for the movement of electrons.
- Cathode – The cathode is the positive electrode of the battery. It’s made from graphite, a porous material with high electrical conductivity.
- Separator – The separator is a thin, porous material that sits between the anode and the cathode. Its purpose is to keep the electrodes from touching each other. This is important to prevent overheating, which could result in the battery catching fire.
- Electrolyte – The electrolyte is a liquid that serves as a conductor of an electric charge. The electrolyte helps move electrons from the anode to the cathode.
- Container – The container or housing holds all of the components of the battery in place. It’s made from a corrosion-resistant material, such as stainless steel.
- Cooling System – The cooling system ensures that the battery does not overheat. This can happen if the battery is overcharged and the temperature of the battery rises.
Battery Cells
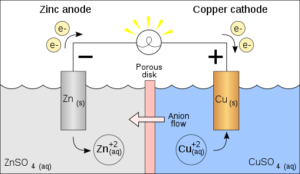
The most important component of the battery is the cell, which is often made from lithium-ion or lead-acid materials. The cell is composed of active materials, electrolytes, and electrodes that are used to store and discharge electricity. The electrode is a conductor that helps to move electrons from one electrode to the other.
The most common electrodes used to make the anode and cathode are lithium and lead. Batteries can be composed of one cell or many cells connected Single-cell batteries are the most common type of electric car battery. Multicell batteries are used in larger-scale storage, such as solar systems or large-scale energy storage systems.
Electronic Parts
The electronic parts of an EV battery include the battery management system (BMS), the charge controller, and the voltage regulator. The BMS is a b16-Monthn electric circuit that’s used to monitor the health of the battery by measuring voltage levels, charging/discharging rates, and temperature.
The BMS can also help to prevent overcharging and over-discharging of the battery. The charge controller is used to charge the battery. It helps to balance the amount of energy used to charge the battery and the amount of energy generated from the grid or solar panel.
The charge controller also measures the amount of current flowing into and out of the battery during charging. The voltage regulator is used to balance the voltage levels of the battery during charging and discharging.
Lead-Acid Batteries
Lead-acid batteries are the oldest type of battery used in electric cars. They are very cheap to produce and are easy to maintain. However, they are not as efficient as other battery types. They also contain toxic materials, such as sulfuric acid.
These types of batteries are typically used in large-scale grid energy storage systems, such as in a commercial or industrial setting. Lead-acid batteries come in both flooded and sealed types, with the flooded type being the most common. Flooded lead-acid batteries are filled with a liquid electrolyte. They are commonly used in electric vehicle systems.
Lithium-Ion Batteries
Lithium is very popular because of the ease with which it can release its electron, which makes it ideal for the electrons to flow between the anode and cathode.
Lithium-ion batteries are very efficient, have a long lifespan, and are capable of being fully charged in less than one hour. They are less expensive than nickel-metal hydride batteries and are used in a wide range of consumer electronics. Currently, hybrid electric vehicles, plug-in hybrid electric vehicles, and electric vehicles used lithium-ion batteries.
Where Do the Materials that Make Up a lithium-ion Battery Come From?
Generally speaking, five minerals are considered essential for Li-ion batteries:
The locations where these materials are mined can originate in many different parts of the world, with China being the major exporter of graphite, which is the most important mineral that comprises the anode for these batteries
Nickel-Metal Hydride Batteries
Nickel-metal hydride batteries are also used in electric cars in both hybrid vehicles and electric vehicles. They are cheaper than lithium-ion batteries and are easier to recycle.
Conclusion
There are many different types of electric car batteries, each with its unique properties and functions. When considering the various types of batteries, it’s important to understand what makes up these different battery types.
Understanding how they function can help you make a more informed decision when purchasing a new electric car battery or an extended-range electric vehicle battery.
When looking for new batteries, make sure to understand their warranties and how they are manufactured to ensure you get the best product possible.
