
Silicon in Its Pure Form
The 14th element in the periodic table, silicon is a grey, shiny metalloid with multiple uses. Besides oxygen, silicon is the second most readily available element on Earth and the 8th most common element found in the universe. Naturally, silicon occurs as a compound, bound up with other elements.
Silicon is one of the seven elements that are known as metalloids, which refers to elements that possess the properties of both metals and nonmetals. This makes it ideal for use in many different industries and is the main component in making alloys (mixing metals with non-metals). Silicon is not only used in the construction industry, but high-tech equipment like computer chips, solar panels, and transistors are all made up of silicon.
The fact that silicon can act as a semiconductor, by allowing control of electrical current, makes it ideal for virtually all electronic equipment.
Facts About Silicon
Silicon is one of the most interesting elements in the periodic table. Some facts about this element are as follows:
- Naturally, silicon is not found in its pure state and is always combined with other elements.
- Over 90% of the Earth’s crust is made up of silicon-containing compounds.
- Most meteorites contain large amounts of silicon.
- On the Mohs scale of hardness, silicon carbide scores an impressive 9-9.5, which is slightly less than a diamond.
- The hardness of silicon compounds makes them an ideal abrasive for industrial use.
- Silicon was first isolated to develop silicon-only crystals in 1854.
- Silicon has a higher density in liquid form as compared to when it is solid.
- Unlike most metals, the conductivity of silicon improves when the temperature increases.
Properties of Silicon
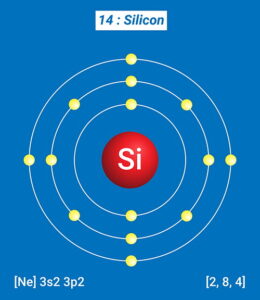
Due to its metalloid nature, silicon does not behave like a typical metal or non-metal but shares the properties of both. Certain factors like temperature and combination with other elements affect its behavior and properties. Some properties of silicon are:
- Silicon is a Semiconductor
Silicon does not behave like a typical metal or nonmetal. This is the reason why this mineral is considered as a semiconductor. It can act as a conductor of electrical current or an insulator depending upon the temperature. As the temperature increases, silicon’s conductivity gets better. - Melting and Boiling Points
Though silicon is not a pure metal, it has a very high melting and boiling point. The melting point of silicon is 1410 degrees Celsius, whereas the boiling point is 2355 degrees Celsius. - Reaction with Other Elements
Pure silicon is highly reactive. Since there are four valence electrons available, silicon can form an ionic or covalent bond by sharing or giving away its electrons. This is the reason why it is not available in its pure form naturally. In its solid form, silicon remains an inert element and does not react with oxygen or water.
Uses of Silicon
The structure and properties of silicon make it a suitable element for a number of industries. Though silicon is hardly used in its pure form, silicon compounds are more commonly used for industrial applications.
Alloy Making
This metal is widely used in making alloys. It is produced at very high temperatures and when heated, it can easily react with other elements like iron. Ferrosilicon is one of the most commonly used silicon alloys and is used in the manufacturing of steel. This alloy of iron and silicon gives hardness and strength to steel. It is also used as the prime deoxidizer in steel manufacturing and helps in removing impurities from the steel.
The aluminum industry also heavily relies on the use of silicon alloys. These alloys are used in welding and manufacturing of molds.

Electronics
One of the most important properties of silicon is that it works as a semiconductor. Its high melting point and ability to conduct electricity make it ideal for its use in the electronics industry.
Prior to its use for electronic devices, silicon is refined in two stages. First, oxygen is removed from the compound, and then it is further refined to produce hyper-pure silicon, which is a semiconductor-grade element. Hyperpure silicon is used in the manufacturing of many electronic devices, including transistors, circuit boards, and microchips which have multiple uses.
A recent milestone in computer technology using silicon-based chips is the invention of quantum computers. These computers can outperform normal computers. Using silicon, these computers can replace normal computers in the near future.
A recent development in medicine is the use of silicon nanoneedles. These are tiny needles that are used for intracellular drug delivery.

Solar Panels
With climatic changes and a high concentration of greenhouse gases, there is much emphasis on the use of renewable sources of energy. Solar energy is one of the most environmentally friendly sources of energy. Using solar panels, we can easily convert the energy of the sun into electricity without damaging the environment.
Most solar cells and solar panels are created using silicon because of its physical and chemical properties. Silicon’s ability to work as a semiconductor makes it the most suitable element for solar panels. However, pure silicon is not used as it is a poor conductor. Silicon is mixed with impurities (also known as doping) so that it can absorb the energy from the sun and convert it into electricity.
Initially, using silicon in the manufacturing of solar panels was an expensive technology. With recent developments in research and technology, silicon solar panels are now affordable for most people.
Nature has provided mankind with all the resources it needs for survival. It’s up to us how we harness and utilize the bounties of nature without damaging the environment.
Summary
The discovery of silicon has had a major impact on how we live our lives every day. Although it is not apparent, it would be hard to imagine how we would live today without the use of silicon.
