Did you ever see the movie “The Incredible Shrinking Man”? If you have, did you ever wonder what would happen to him when he gets so small that he would be the size of an atom? And if so, could he get any smaller?
Maybe we have the answer because atoms are particles that exist in nature and cannot be broken down into smaller components. Everything we see around us is made of atoms, from tables and chairs to people and pandas.
What Makes Up the Atom?
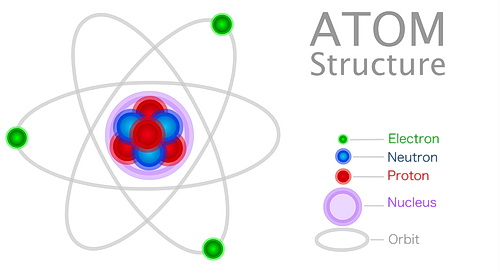
Comparatively speaking, atoms contain mostly space but don’t let that fool you into thinking they are not important. The components of the atom and what makes up the atom are fundamental to our understanding of how matter is assembled. That includes living organisms, both here on Earth and elsewhere.
Now let’s talk about the components. A typical atom consists of a nucleus in its center. This nucleus contains neutrons and protons (together they’re called nucleons). Protons have a positive charge. Neutrons have neither positive nor negative charges. They are ‘neutral’.
Surrounding the nucleus are electrons, which are bodies outside of the nucleus and orbit around it, the same as our planets orbit their sun. Besides the size difference in this comparison, the only major difference is that the planets orbit the sun because of gravity, and electrons orbit their nucleus because of magnetism.
Note: The above scenario is simplified to envision the structure of the atom. The real fact is that electrons do not orbit the nucleus as the planets do. Their actions are more complex than that. See our article on Quantum Theory for a better understanding of how electrons maneuver around the atom’s nucleus.
The Electron
An electron orbits the nucleus of the atom. They are negatively charged particles. The electrons are the only particles outside of the atom’s nucleus.
Neutral Atoms
A neutral atom doesn’t have any charge, so it doesn’t interact with other atoms. You can think of it as a bag of protons, neutrons, and electrons that just float around in space. Most neutral atoms are made up of an equal number of protons, neutrons, and electrons. For example, hydrogen has one proton, one neutron, and one electron. Helium atoms have two protons, two neutrons, and two electrons. This is why we usually refer to these atoms as neutral.
The Proton
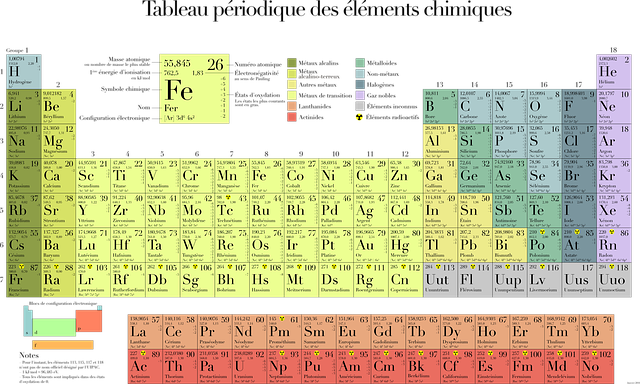
Protons are mainly found in the nucleus, although a few may be found in the outer electron orbit. The number of protons in an atom is what makes it what it is. For example, the elements in the periodic table have numbers associated with them. The number on the upper right corner defines its atomic number; that is, it tells us the number of protons in that element. Atomic weight is the number of protons and neutrons together.
Neutrons
The neutron’s only job is to protect the proton from becoming too positively charged. It doesn’t matter if the atom has too many or too few neutrons; it’s fine either way. The neutron doesn’t interact with electrons or anything else outside the nucleus, so it’s usually just along for the ride.
The valence electrons (see below) of an atom are the electrons that are available to form chemical bonds with other atoms. In general, valence electrons are those that can be shared in their atomic orbitals.
Each main group element has a fixed number of valence electrons, which makes it easier to predict how likely an element is to react with another one and whether or not a given element can act as a reducing agent. Combining all of this information, we can deduce the oxidation state (or valence) of each element and predict whether or not they will react with one another based on these findings. Let’s take a closer look at what these valence electrons are and what role they play in chemical reactions.
Ions
Any time an atom loses or gains an electron, it becomes charged. If it loses an electron, it becomes positively charged. because there are more protons in the atom than electrons. If it gains an electron, it becomes negatively charged.
When atoms gain or lose an electron, they can bond together with other ions to form other elements; thereby creating a new atom or molecule.
Note: Regardless of the number of electrons or protons that are lost or gained, the ‘makeup’ of the atom is associated with the number of protons that are in the atom, as designated in the upper right corner of each element of the periodic table.
So What are Valence Electrons?
These are electrons that are in the outermost shell of an atom and if these atoms have less than 8 electrons in this shell, they will look to find other atoms to bond with so that their outer shells can reach 8 electrons.
This is the Octet Rule, which states that atoms with less than 8 electrons in their outer shell will tend to bond with other atoms so that they can share their valence shells and have eight electrons, hence, the “octet (8)” rule.
From our explanation of ions above, it is these electrons that are participating in the chemical reactions (bonding) with other atoms, since they are the farthest away from the nucleus and thus, have the least magnetic force attached to them. In other words, can easily get detached or pulled from a nearby atom.
So, it is these electrons that are the ones that cause the sharing of electrons with other atoms.
Valence Proximity
The electrons that are closer to the nucleus are referred to as core electrons since they aren’t as likely to participate in chemical reactions. The core electrons are essential to the existence of an atom because without them the atom would collapse in on itself. However, they’re not as likely to be involved in chemical reactions with other atoms because they’re so close to the nucleus.
Valence Summary
The valence electrons are the outermost electrons in an atom that is available to form chemical bonds with other atoms. The number of valence electrons for each element is fixed, and we can use the location of these electrons to predict how likely it is for an atom to bond with another. The more stable the core electrons are, the more difficult it will be for an atom to accept its electrons. If you’re studying chemistry and need to understand how chemical reactions work, it’s important to understand what valence electrons are and how they are used during chemical reactions.
All Together Now
The negative charge of the electrons and the positive charge of the protons are what maintain the orbit of the electrons around the nucleus. This is referred to as an electrostatic charge or electromagnetic force, or to put it another way, it is the attraction of the positive charge from the negative charge of the electrons that causes this orbit to exist.
Now, let’s drill down to more specifics of the atom’s components and how their respective charges make up different types of atoms.
Conclusion
Atoms are the smallest particles of matter that cannot be broken down into smaller components. Everything we see around us is made of atoms. Atoms are mostly empty spaces, but they’re fundamental to our understanding of how matter works. A typical atom consists of a nucleus with neutrons and protons (together called nucleons) inside it, as well as electrons that orbit the nucleus. The electrons have a negative charge; the nucleons have a positive charge.
Neutral atoms are made up of an equal number of protons, neutrons, and electrons. Ionic compounds are made up of positively charged ions and negatively charged electrons, and they have a strong attraction to other atoms and molecules. Electrons are negatively charged particles that orbit the nucleus, making them useful tools. Atoms are the building blocks of everything in the universe, and they are fundamental to our understanding of how matter works.
